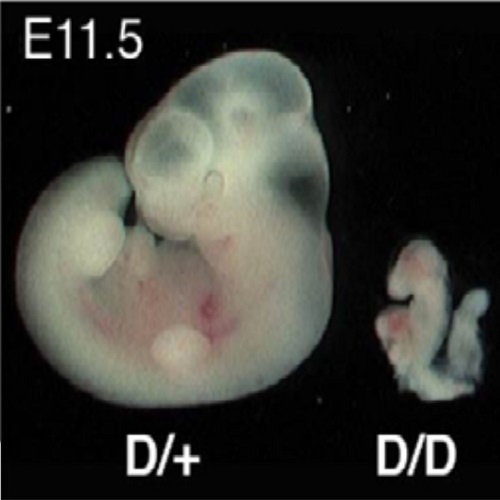
The H3K4 methyltransferase Setd1a is first required at the epiblast stage, whereas Setd1b becomes essential after gastrulation.
Histone 3 lysine 4 (H3K4) methylation is a universal epigenetic mark. In mammals, there are six H3K4 methyltransferases related to yeast Set1 and fly Trithorax, including two orthologs of Set1: Setd1a and Setd1b. Here we show that mouse Setd1a is required for gastrulation, whereas Setd1b-deficient embryos survive to E11.5 but are grossly retarded. Setd1a knockout embryos implant but do not proceed past the epiblast. Furthermore, Setd1a is not required until the inner cell mass has formed, at which stage it has replaced Mll2 as the major H3K4 methyltransferase. Setd1a is required for embryonic, epiblast and neural stem cell survival and neural stem cell reprogramming, whereas Setd1b is dispensable. Deletion of Setd1a in embryonic stem cells resulted in rapid losses of bulk H3K4 methylation, pluripotency gene expression and proliferation, with G1 pileup. Setd1b overexpression could not rescue the proliferation defects caused by loss of Setd1a in embryonic stem cells. The precise developmental requirement for Setd1a suggests that gastrulation is regulated by a switch between the major H3K4 methyltransferases.
Back to list