MinCDE exploits the dynamic nature of FtsZ filaments for its spatial regulation.
In Escherichia coli, a contractile ring (Z-ring) is formed at midcell before cytokinesis. This ring consists primarily of FtsZ, a tubulin-like GTPase, that assembles into protofilaments similar to those in microtubules but different in their suprastructures. The Min proteins MinC, MinD, and MinE are determinants of Z-ring positioning in E. coli. MinD and MinE oscillate from pole to pole, and genetic and biochemical evidence concludes that MinC positions the Z-ring by coupling its assembly to the oscillations by direct inhibitory interaction. The mechanism of inhibition of FtsZ polymerization and, thus, positioning by MinC, however, is not understood completely. Our in vitro reconstitution experiments suggest that the Z-ring consists of dynamic protofilament bundles in which monomers constantly are exchanged throughout, stochastically creating protofilament ends along the length of the filament. From the coreconstitution of FtsZ with MinCDE, we propose that MinC acts on the filaments in two ways: by increasing the detachment rate of FtsZ-GDP within the filaments and by reducing the attachment rate of FtsZ monomers to filaments by occupying binding sites on the FtsZ filament lattice. Furthermore, our data show that the MinCDE system indeed is sufficient to cause spatial regulation of FtsZ, required for Z-ring positioning.
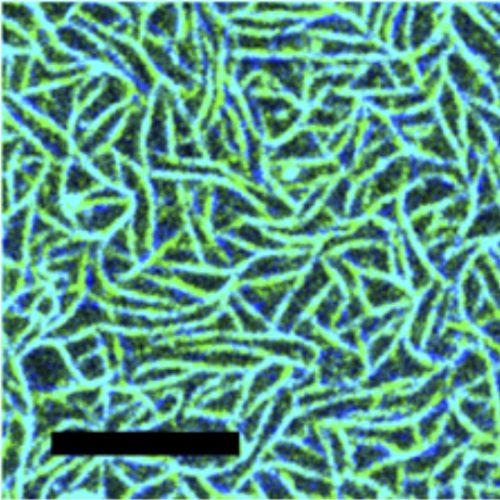
- Proc. Natl. Acad. Sci. U.S.A. 2014 Apr 1;111(13):E1192-200
- 2014
- Biophysics
- 24707052
- PubMed
Enabled by:
