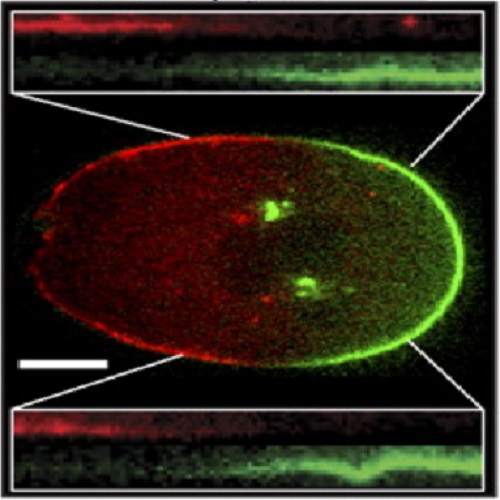
PAR proteins diffuse freely across the anterior-posterior boundary in polarized C. elegans embryos.
Polarization of cells by PAR proteins requires the segregation of antagonistic sets of proteins into two mutually exclusive membrane-associated domains. Understanding how nanometer scale interactions between individual PAR proteins allow spatial organization across cellular length scales requires determining the kinetic properties of PAR proteins and how they are modified in space. We find that PAR-2 and PAR-6, which localize to opposing PAR domains, undergo exchange between well mixed cytoplasmic populations and laterally diffusing membrane-associated states. Domain maintenance does not involve diffusion barriers, lateral sorting, or active transport. Rather, both PAR proteins are free to diffuse between domains, giving rise to a continuous boundary flux because of lateral diffusion of molecules down the concentration gradients that exist across the embryo. Our results suggest that the equalizing effects of lateral diffusion are countered by actin-independent differences in the effective membrane affinities of PAR proteins between the two domains, which likely depend on the ability of each PAR species to locally modulate the membrane affinity of opposing PAR species within its domain. We propose that the stably polarized embryo reflects a dynamic steady state in which molecules undergo continuous diffusion between regions of net association and dissociation.
- J. Cell Biol. 2011 May 2;193(3):583-94
- 2011
- Cell Biology
- 21518794
- PubMed
Enabled by:
