One-step purification of assembly-competent tubulin from diverse eukaryotic sources.
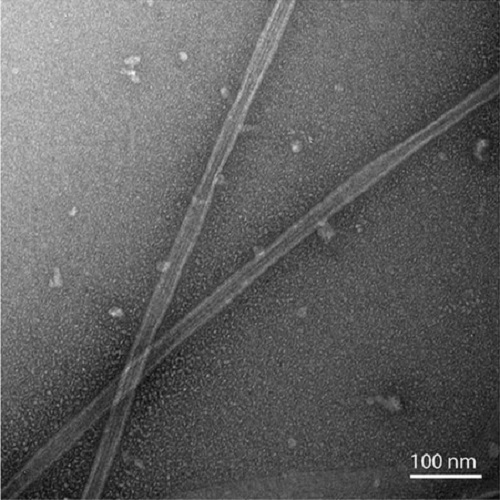
We have developed a protocol that allows rapid and efficient purification of native, active tubulin from a variety of species and tissue sources by affinity chromatography. The affinity matrix comprises a bacterially expressed, recombinant protein, the TOG1/2 domains from Saccharomyces cerevisiae Stu2, covalently coupled to a Sepharose support. The resin has a high capacity to specifically bind tubulin from clarified crude cell extracts, and, after washing, highly purified tubulin can be eluted under mild conditions. The eluted tubulin is fully functional and can be efficiently assembled into microtubules. The method eliminates the need to use heterologous systems for the study of microtubule-associated proteins and motor proteins, which has been a major issue in microtubule-related research.
- Mol. Biol. Cell 2012 Nov 19;23(22):4393-401
- 2012
- Imaging Technologies Development
- 22993214
- PubMed
Enabled by:
