Janus face-like effects of Aurora B inhibition: antitumoral mode of action versus induction of aneuploid progeny.
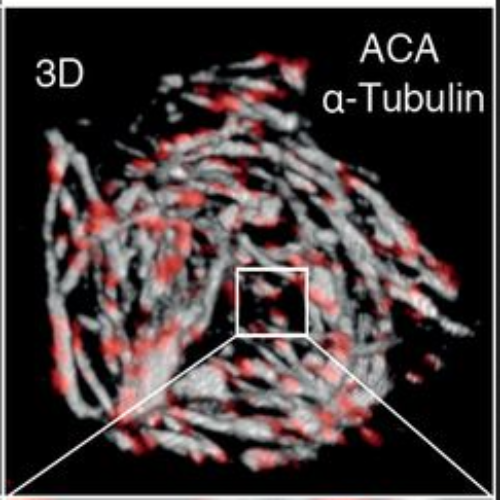
The mitotic Aurora B kinase is overexpressed in tumors and various inhibitors for Aurora B are currently under clinical assessments. However, when considering Aurora B kinase inhibitors as anticancer drugs, their mode of action and the role of p53 status as a possible predictive factor for response still needs to be investigated. In this study, we analyzed the effects of selective Aurora B inhibition using AZD1152-HQPA/Barasertib (AZD1152) on HCT116 cells, U87-MG, corresponding isogenic p53-deficient cells and a primary glioblastoma cell line. AZD1152 treatment caused polyploidy and non-apoptotic cell death in all cell lines irrespective of p53 status and was accompanied by poly-merotelic kinetochore-microtubule attachments and DNA damage. In p53 wild-type cells a DNA damage response induced an inefficient pseudo-G1 cell cycle arrest, which was not able to halt ongoing endoreplication of cells. Of note, release of tumor cells from AZD1152 resulted in recovery of aneuploid progenies bearing numerical and structural chromosomal aberrations. Yet, AZD1152 treatment enhanced death receptor TRAIL-R2 levels in all tumor cell lines investigated. A concomitant increase of the activating natural killer (NK) cell ligand MIC A/B in p53-deficient cells and an induction of FAS/CD95 in cells containing p53 rendered AZD1152-treated cells more susceptible for NK-cell-mediated lysis. Our study mechanistically explains a p53-independent mode of action of a chemical Aurora B inhibitor and suggests a potential triggering of antitumoral immune responses, following polyploidization of tumor cells, which might constrain recovery of aneuploid tumor cells.
- Carcinogenesis. 2016 Oct;37(10):993-1003
- 2016
- Medical Biology
- 27515963
- PubMed
Enabled by:
