Secreted protein Del-1 regulates myelopoiesis in the hematopoietic stem cell niche.
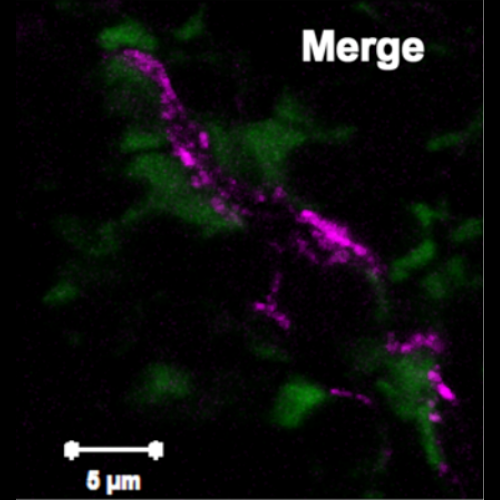
Hematopoietic stem cells (HSCs) remain mostly quiescent under steady-state conditions but switch to a proliferative state following hematopoietic stress, e.g., bone marrow (BM) injury, transplantation, or systemic infection and inflammation. The homeostatic balance between quiescence, self-renewal, and differentiation of HSCs is strongly dependent on their interactions with cells that constitute a specialized microanatomical environment in the BM known as the HSC niche. Here, we identified the secreted extracellular matrix protein Del-1 as a component and regulator of the HSC niche. Specifically, we found that Del-1 was expressed by several cellular components of the HSC niche, including arteriolar endothelial cells, CXCL12-abundant reticular (CAR) cells, and cells of the osteoblastic lineage. Del-1 promoted critical functions of the HSC niche, as it regulated long-term HSC (LT-HSC) proliferation and differentiation toward the myeloid lineage. Del-1 deficiency in mice resulted in reduced LT-HSC proliferation and infringed preferentially upon myelopoiesis under both steady-state and stressful conditions, such as hematopoietic cell transplantation and G-CSF- or inflammation-induced stress myelopoiesis. Del-1-induced HSC proliferation and myeloid lineage commitment were mediated by β3 integrin on hematopoietic progenitors. This hitherto unknown Del-1 function in the HSC niche represents a juxtacrine homeostatic adaptation of the hematopoietic system in stress myelopoiesis.
- Clin Invest. 2017 Oct 2;127(10):3624-3639.
- 2017
- Cell Biology
- 28846069
- PubMed
Enabled by:
