Targeted knock-in of CreER T2 in zebrafish using CRISPR/Cas9.
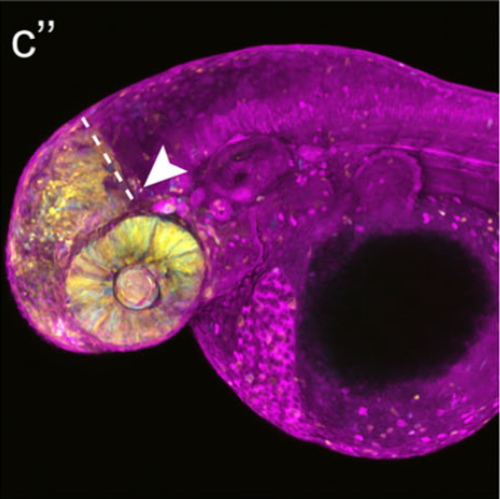
New genome-editing approaches, such as the CRISPR/Cas system, have opened up great opportunities to insert or delete genes at targeted loci and have revolutionized genetics in model organisms like the zebrafish. The Cre-loxp recombination system is widely used to activate or inactivate genes with high spatial and temporal specificity. Using a CRISPR/Cas9-mediated knock-in strategy, we inserted a zebrafish codon-optimized CreER T2 transgene at the otx2 gene locus to generate a conditional Cre-driver line. We chose otx2 as it is a patterning gene of the anterior neural plate that is expressed during early development. By knocking in CreER T2 upstream of the endogenous ATG of otx2, we utilized this gene's native promoter and enhancer elements to perfectly match CreER T2 and endogenous otx2 expression patterns. Next, by combining this novel driver line with a Cre-dependent reporter line, we show that only in the presence of tamoxifen can efficient Cre-loxp-mediated recombination be achieved in the anterior neural plate-derived tissues like the telencephalon, the eye and the optic tectum. Our results imply that the otx2:CreER T2 transgenic fish will be a valuable tool for lineage tracing and conditional mutant studies in larval and adult zebrafish.
Back to list